Abstract
Background
Expanding availability of novel agents for treating MM has transformed management strategies, particularly for relapsed/refractory (R/R) disease. Since 2013, experienced MM physicians from leading academic institutions and cancer centers (experts) have annually updated an online tool designed to provide HCPs with treatment recommendations for specific patient cases. Previous reports from our tool analyses have shown yearly changes in treatment patterns among experts but a multiyear delay by HCPs in the adoption of many expert-recommended treatment strategies into their practice.
Methods
To develop the tool, 5 MM experts provided treatment recommendations for hundreds of case scenarios across induction, maintenance, and R/R disease settings. To use the tool, HCPs seeking treatment consultation entered patient and disease factors with a planned treatment for that specific patient using dropdown menus and received 5 expert recommendations for their case. HCPs were asked to indicate the impact of expert recommendations on their planned treatment. This report includes analysis and comparison of intended treatment of HCPs for 790 cases entered into the 2017 iteration of tool with expert recommendations for those patient cases. Treatment trends observed in 2017 will also be compared with those from previous years.
Results
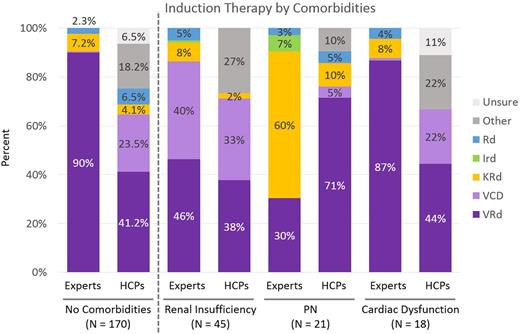
Consensus (≥ 3 of 5 experts choosing the same treatment) among experts has been increasing for induction therapy since 2013. In ASCT-eligible patients, regardless of any other factors, experts recommended bortezomib/lenalidomide/dexamethasone (VRd) in 75% of tool cases in 2017, up from 58% in 2016 and 45% in 2013 and 2014. By comparison, HCPs used VRd for 44% of ASCT-eligible patient cases in 2017 and only 19% in 2016. Expert recommendation of VRd for 66% (vs 48% of HCPs) of ASCT-ineligible cases in 2017 also increased from 38% (vs 18% of HCPs) in 2016. Expert recommendations for induction therapy changed most for cases with varying comorbidities, and data from the 2017 tool are shown in the figure below. In patients without specific comorbidities or with cardiac dysfunction only, experts recommended VRd for 87% to 90% of these cases (vs 41% to 44% of HCPs). In cases involving peripheral neuropathy, experts recommended carfilzomib/lenalidomide/dexamethasone (KRd) for 60% and VRd for 30% compared with HCPs selecting KRd for 10% and VRd for 71% of those cases.
In the R/R setting, the novelty and broad range of available regimens are reflected in the lack of consensus in treatment recommendations by both the experts as well as the planned treatment of HCPs. In 304 R/R case scenarios, only 36 cases (12%) had an exact expert treatment consensus and 63 cases (21%) had 2 pairs of experts agreeing on the same treatment recommendations. However, overall treatment strategies were similar among experts, with use of triplet regimens including novel agents increasing in 2017. Experts recommended Kd or daratumumab monotherapy for 40% of cases refractory to lenalidomide in 2016. In 2017, experts no longer recommended monotherapy or doublet therapy for R/R cases in this setting. In 2017, the experts recommended triplet regimen therapy for 87% to 97% of cases involving bortezomib-refractory or lenalidomide/bortezomib-refractory patients, and each of the recommendations included either carfilzomib, ixazomib, daratumumab, or elotuzumab compared with only 55% to 60% of their recommendations including triplet therapy with one of these agents in 2016. Again, variation was observed in the intended treatment of HCPs compared with the expert recommendations for R/R cases. In 2017, the intended treatment of HCPs did not match any of the experts' recommendations for 45% of the R/R cases entered into the tool.
Conclusions
Analysis of cases entered into our expert-developed treatment decision tool shows that a wide gap remains between expert treatment recommendations and the intended treatment plans of HCPs for many of the same MM case scenarios. Experts had nearly full consensus on choice of induction therapy; however, differences between expert recommendations in R/R MM are due to availability of multiple triplet-based treatment options in this setting. In addition, newly approved therapies continue to have a strong impact on experts' recommendations as they quickly integrate new agents into their treatment approaches. A detailed analysis of expert and HCP MM practice trends will be presented.
Kumar: Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Skyline: Honoraria; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding. Lentzsch: Caelum Biosciences: Other: leadership position and stock; BMS: Consultancy; Amgen: Consultancy. Anderson: Gilead Sciences: Membership on an entity's Board of Directors or advisory committees; Oncopep: Other: scientific founder; C4 Therapeutics: Other: scientific founder; Millenium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; MedImmune: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal